- Home
- SURGERY
- Breast Surgery
- Breast Implants
BREAST IMPLANTS IN PORTLAND, OR
DISCOVER YOUR AUGMENTATION OPTIONS FOR VANCOUVER, SALEM, AND ALL OF OREGON
If you are considering breast augmentation or a breast implant exchange procedure, one of the most important decisions you will ultimately make is the type of breast implants that are best for your ultimate goals. Board-certified plastic surgeons Kathleen Waldorf, MD, and Rachel Streu, MD offer a range of options when it comes to breast implants for Portland, OR, area patients of the Waldorf Center, including size, shape, and type. The selection helps you add volume to the breasts with beautiful, natural-looking results.
YOUR CONSULTATION
 Our experienced plastic surgery team wants you to be fully educated about all aspects of breast surgery. During your initial consultation, our plastic surgeons will meet with you to talk about your goals and answer any questions you may have. They will also tell you what you can expect before, during, and after treatment. Please feel free to bring in pictures of looks that you do or do not like to help us understand your aesthetic tastes. We will also conduct a 3-D image simulation that can give you an idea of the potential results of breast augmentation. The image can be modified with different implant sizes so that you can see a number of potential outcomes. Once you have been examined by our surgeon and you have explored some of the possibilities, they will talk more with you about which option may be the best for your needs.
Our experienced plastic surgery team wants you to be fully educated about all aspects of breast surgery. During your initial consultation, our plastic surgeons will meet with you to talk about your goals and answer any questions you may have. They will also tell you what you can expect before, during, and after treatment. Please feel free to bring in pictures of looks that you do or do not like to help us understand your aesthetic tastes. We will also conduct a 3-D image simulation that can give you an idea of the potential results of breast augmentation. The image can be modified with different implant sizes so that you can see a number of potential outcomes. Once you have been examined by our surgeon and you have explored some of the possibilities, they will talk more with you about which option may be the best for your needs.
SHOULD YOU GET BREAST IMPLANTS?
Deciding whether or not to augment your breasts with implants is a significant choice, and we want you to feel confident with the path you choose. With that in mind, the Waldorf Center team put together a “patient decision checklist” featuring variables to consider, an explanation of risks, recommendations for follow-up care, and more. +
Patient Decision Checklist – Breast Implants
To the patient considering breast implants filled with saline or silicone gel intended for breast augmentation or breast reconstruction: The review and understanding of this document is a critical step in making the decision whether you should choose breast implant surgery. You should learn about breast implants and then carefully consider the benefits and risks associated with breast implants and breast implant surgery before you make that decision. This form lists important risks, including those known or reported to be associated with the use of the device based on information from clinical trials, scientific literature, and reports from patients who have undergone device placement.
This Patient Decision Checklist is intended to supplement the additional patient information documents that should be provided to you by your physician. You should receive patient information documents that include important information about your specific breast implant, as well as a boxed warning and Patient Decision Checklist. After reviewing the information in the patient information documents for the specific implant that will be used, please read and discuss the items in this checklist carefully in consultation with your physician. You should place your initials in the location provided next to each item to indicate that you have read and understood the item. Your full signature at the end of this document confirms that you have read the materials and that your physician has answered all questions to your satisfaction.
Considerations for a Candidate for Successful Breast Implantation
I understand that I am not a candidate for breast implants if any of the following situations applies to me:
- I have an active infection anywhere in my body;
- I have an existing cancer or pre-cancer of my breast tissue that has not been adequately treated; or
- I am pregnant or nursing.
I understand that if I have any of the following conditions, I may be at a higher risk for a poor surgical outcome:
- Medical condition that affects my body’s ability to heal (e.g., diabetes, connective tissue disorder);
- Active smoker or a former smoker;
- Currently taking drugs that weaken the body’s natural resistance to disease, such as steroids and chemotherapy drugs (e.g., prednisone, tacrolimus, sirolimus, mycophenolate, azathioprine, cyclosporine, methotrexate, chlorambucil, leflunomide, or cyclophosphamide);
- History of chemotherapy or planned chemotherapy following breast implant placement;
- History of radiation therapy or planned radiation following breast implant placement;
- Conditions that interfere with wound healing or blood clotting (e.g., hemophilia, von Willebrand disease, factor V Leiden, hyperhomocysteinemia, protein C deficiency, antithrombin III deficiency, or systemic lupus erythematosus); or
- Reduced blood supply to the breast tissue.
I understand the following conditions have not been adequately studied to determine whether the conditions put me at higher risk:
- Autoimmune disease (e.g., Hashimoto’s, Lupus, Rheumatoid Arthritis) or family history of autoimmune disease (breast implant premarket clinical studies have not evaluated the safety of breast implants in patients with autoimmune disease);
- Clinical diagnosis of depression or other mental health disorder (including body dysmorphic disorder or eating disorder); or
- Have other products permanently implanted in the breast.
Patient Initials: __________
Risks of Breast Implant Surgery
I understand that there are risks of undergoing breast implant surgery. I understand that risks of undergoing breast implant surgery may include:
- breast pain (reported in up to 11.7% of patients1),
- skin or nipple areola sensitivity changes or loss (nipple complications reported in up to 6.3% of patients1 and breast/skin sensation changes reported in up to 2.2% of patients1),
- asymmetry (reported in up to 23.2% of patients1),
- impact of aging or weight change on size and shape of breast (ptosis reported in up to 4.9% of patients1),
- infection requiring possible removal of implant (reported in up to 3.2% of patients1),
- swelling (reported in up to 9.2% of patients1),
- scarring (hypertrophic scarring reported in up to 6.6% of patients1),
- fluid collections (seroma)(reported in up to 6.7% of patients1),
- hematoma (reported in up to 2.1% of patients1),
- tissue death of breast skin or nipple (tissue/skin necrosis reported in up to 2.3% of patients1),
- inability to breast feed (lactation complications reported in up to 30% of patients1),
- complications of anesthesia (may occur but specific rates are not publicly available in the Allergan Core Study),
- bleeding (may occur but specific rates are not publicly available in the Allergan Core Study),
- chronic pain (may occur but specific rates are not publicly available in the Allergan Core Study),
- damage to surrounding tissue, such as muscle, nerves, and blood vessels (may occur but specific rates are not publicly available in the Allergan Core Study), and
- impact on imaging of breast tissue (may occur but specific rates are not publicly available in the Allergan Core Study).
My physician has discussed these risks and has provided me with the patient information documents (including the boxed warning) with information on the types of risks that are possible and expected rates of occurrence.
My physician has discussed the potential use of other implanted products during my breast implant surgery. My physician has also discussed the risks and benefits of using these implanted products and their planned surgical approach.
Patient Initials: __________
Risk of Cancer – Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA- ALCL)
I understand that breast implants are associated with the development of a type of cancer of the immune system called Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL). Information regarding the number of medical device reports of BIA-ALCL can be found on FDA’s website.2
As of July 2019, literature reports various estimates for the incidence of BIA-ALCL. These estimated incidence rates range from a high of 1 per 3,817 patients to 1 in 30,000. (Clemens et al, 2017, Loch-Wilkinson 2017 et al, De Boer et al, 2018).
I have received information regarding the overall incidence rates of BIA-ALCL and the rates as they pertain to my specific breast implant. I understand that this cancer has been reported more frequently for textured breast implants, but patients with smooth surfaced implants have also been diagnosed.
I understand that patients with breast implants have a risk of developing BIA-ALCL within the scar tissue and fluid surrounding the breast implant.
I understand that BIA-ALCL typically takes several years to develop after implantation, but cases have been reported as early as within one year. Typical symptoms to be aware of may include: breast tightness, pain, lumps, or swelling of the breast months or years after I receive my implants.
I understand that treatment for BIA-ALCL involves an operation to remove the implants and the surrounding scar tissue capsule. Based on the stage of the cancer at diagnosis, some patients have required chemotherapy or radiation. While BIA-ALCL typically responds well to therapy, some patients have died from BIA-ALCL. Diagnosis and treatment may be at my own expense and is not always covered by insurance.
Patient Initials: __________
Systemic Symptoms
I understand that some patients who have received breast implants have reported a variety of systemic symptoms including joint pain, fatigue, rash, memory loss, and “brain fog” that some patients have called breast implant illness. While the causes of these symptoms are unclear, some patients have reported relief of these symptoms with removal of their implants and surrounding scar capsule; however, not all patients may experience improvement in their symptoms. Researchers are working to better understand the possible link between breast implants and these symptoms.
I also understand that some patients with breast implants have reported health problems in their children after birth or breastfeeding. A causal link has not been established between breast implants and these reported health problems in children and more research is needed. I understand that breast implants and breast surgery may interfere with my ability to successfully breastfeed.
Patient Initials: __________
Breast-Implant Specific Risks
I understand that a breast implant is NOT a lifetime device and the longer I have my implants, the more likely I am to experience a complication and the more likely I am to need a reoperation requiring the replacement or removal of my breast implant. As many as 32.4% of women who received breast implants for augmentation had their implants removed within 10 years, but my implants may last for a shorter or longer period of time. (The percentage reported is from the 10-year Core Clinical Study for NATRELLE® Silicone gel-filled breast implants. The rate specified represents the largest reported cumulative 10-year rate across all groups of augmentation patients in the study (both primary and revision)).
I understand that my breast implant may rupture or leak at any time, and that the longer I have my implants, the more likely I am to experience a complication such as rupture. I understand that gel bleed (small quantities of gel diffusing from the implant shell) of silicone gel-filled implants may occur. I understand that if I have a saline-filled implant, my breast may deflate in appearance if there is a rupture or leakage of the saline.
I understand that if I have a silicone gel-filled breast implant, I or the physician may not be able to tell on physical exam whether my implant has ruptured or is leaking silicone gel. Because rupture or leakage of silicone gel-filled breast implants is difficult to detect, I understand that periodic imaging evaluation is recommended for screening of silicone gel-filled breast implant rupture. It is recommended that I have periodic imaging of my silicone gel-filled breast implants to screen for implant rupture regardless of whether my implants are for cosmetic augmentation or reconstruction. These recommendations do not replace other additional imaging that may be required depending on my medical history or circumstances (i.e., screening mammography for breast cancer).
Even if I have no symptoms, I should have regular imaging evaluations as described in the “Recommended Follow-Up” section below. These imaging evaluations may not detect all ruptures or leaks, and the expense may not be covered by my medical insurance.
I understand that there are rare case reports of silicone migrating from breast implants into tissues (e.g., chest wall, lymph nodes under the arm) and organs (e.g., liver, lungs). It may not be possible to remove migrated silicone.
I understand that all breast implants can affect mammography and breast exams, which could potentially delay the diagnosis of breast cancer. Mammography can also cause the breast implant to rupture or leak. I should tell the mammography technician if I have breast implants.
I understand that the long-term risks of breast implants may include:
- painful or tightening of scar tissue (capsule) around my implant (capsular contracture III/IV) (reported in up to 28.7% of patients1),
- rupture or leaking of the implant (reported in up to 35.4% of patients1),
- wrinkling of the implant (wrinkling/rippling reported in up to 10.2% of patients1),
- visibility of the implant edges (implant palpability/visibility reported in up to 6.7% of patients1),
- shifting of the implant (implant malposition reported in up to 13.3% of patients1), or
- reoperation (reported in up to 71.5% of patients1).
I understand that I will receive a Device Identification Card after my surgery that has information on each of my specific implants. I understand that it is important for me to keep each card in case, at some time in the future, I or my physician need to know what kind of implant I received many years later.
I understand that breast implant manufacturing requires the use of chemicals and heavy metals. I understand that most of these chemicals stay inside the shell of the implant. Small quantities may diffuse (gel bleed) through the implant shell of silicone gel-filled implants, even if the implant is intact and not ruptured or leaking. A list of the components, chemicals, and heavy metals is available in the section entitled, “NATRELLE® Silicone-Filled/Saline-Filled Breast Implant Device Materials” of the patient information document.
Patient Initials: __________
Recommended Follow-up
Even if I have no symptoms, I should have my first ultrasound or MRI at 5-6 years after my initial implant surgery and then every 2-3 years thereafter. If I have symptoms or uncertain ultrasound results for breast implant rupture, an MRI is recommended.
I understand that for as long as I have my breast implant(s), I will need routine and regular follow-up with my physician, for examination of my breast implant(s) as well as to discuss any updates regarding breast implant issues.
National Breast Implant Registry (NBIR): I understand and have discussed with my physician that there is a National Breast Implant Registry where information regarding my health and breast implant information can be entered. The NBIR may help understand the long-term safety and performance of breast implants.
Patient Registry and Outcomes For breast Implants and anaplastic large cell Lymphoma (ALCL) etiology and Epidemiology (PROFILE): I understand and have discussed with my physician that there is a registry (PROFILE) where information is collected to better understand BIA-ALCL in patients with breast implants.
Patient Initials: __________
Questions for My Physician
I have had the opportunity to ask my physician questions about his or her experience, medical degree, specialty of training, and credentials. I understand that breast implants have associated procedural risks and should only be used by physicians who are appropriately trained.
Patient Initials: __________
Options Following Mastectomy
I understand that breast reconstruction is an elective procedure, which I can choose to do or not. I understand that I may choose not to have breast reconstruction (“going flat”) and may choose to use an external prosthesis in my bra to look like I have a breast when wearing clothes.
I understand the surgical options for breast reconstruction, including the use of a breast implant and the use of my own tissue (“autologous reconstruction”). I understand that if my breast implants are ever removed, I may be left with dimpling, chest wall concavity, puckering, or sagging of my breasts or skin.
I understand that more surgeries may be necessary in the future due to complications or to remove or replace the breast implants. I have discussed all of the options for breast reconstruction with my surgeon, including whether I am a candidate and the benefits and risks of each, and I believe that breast reconstruction with a breast implant is the best option for me.
Patient Initials: __________
Breast Augmentation Options
I understand that breast augmentation is an elective procedure to increase the size of my breasts. I understand that breast augmentation may result in permanent changes to my breast tissue and if my implants are ever removed, I may be left with an unsatisfactory appearance, changes to the size and shape of my breasts, including but not limited to dimpling, chest-wall concavity, puckering, sagging, or different incision size or location. If I am an augmentation patient, any additional surgeries or medical procedures will likely be at my own expense.
Patient Initials: __________
OPTIONS FOR BREAST IMPLANTS IN PORTLAND, OR
Breast implants come in a variety of sizes, textures, and shapes. You can also choose from a number of different profile ranges from low to moderate to high depending on which offers the best amount of forward projection for your goals. There are only two major types of breast implants: silicone and saline. The best options for your needs will depend on which breast implants are ideal for your unique body type and which implants can ultimately meet your goals with natural-looking results. Keep in mind that when you add volume to the breasts, it is as much about enhancing the proportions of your breasts as it is about making them larger. The best choices of breast implants are often those that not only add fullness to the breasts but also serve as a beautiful complement to the overall proportions of your figure.
CONTACT US
Learn more about breast implants® at Portland, OR′s Waldorf Center for Plastic Surgery. Call 503-646-0101 or 1-800-310-7901, or schedule a visit online.
Silicone Breast Implants
Silicone breast implants are made of a silicone outer shell and filled with a silicone gel. These implants are known to be less prone to rippling, and many women choose them for breast augmentation because they often yield results that look and feel very natural. Women with thin body frames can often benefit from silicone breast implants. These implants are approved by the FDA for breast augmentation procedures in women who are at least 22 years of age, and for breast reconstruction procedures for patients of any age.
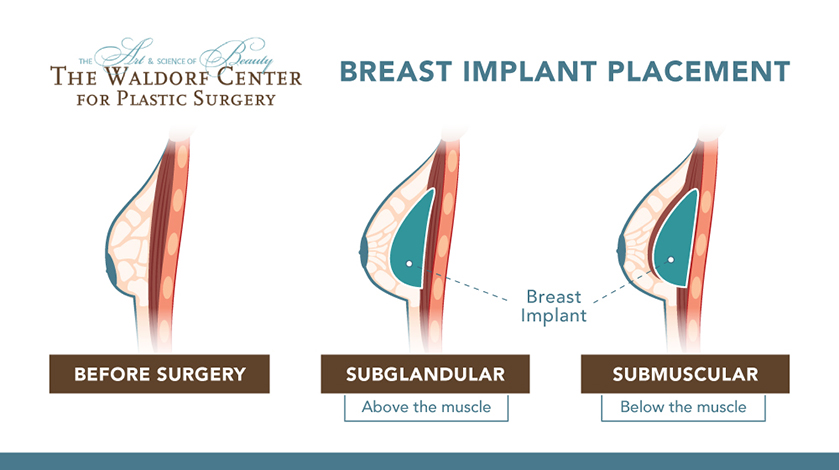
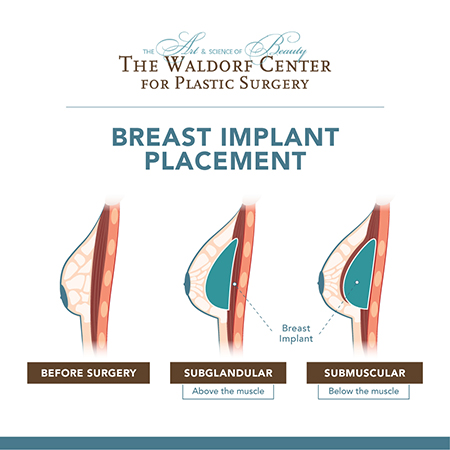
Patients considering breast Implants at Portland’s Waldorf Center have two options for implant placement: subglandular (above the muscle) and submuscular (below the muscle). A subglandular placement refers to when the implant is placed between the breast tissue and the pectoral muscle. Submuscular placement is when the implant is placed beneath both the breast and muscle tissue. Your plastic surgeon will work with you to determine the ideal placement for you. An inframammary incision, which is made directly below the breast in the bottom crease between the breast and the chest wall, will be used for breast augmentation.
Saline Breast Implants
Saline breast implants are composed of a silicone outer shell encasing a saline (salt-water) solution. Saline implants are the most common type of implant in use, and they have helped millions of women across the globe enhance the size of their breasts with natural-looking results. The FDA has approved the use of saline breast implants for breast augmentation procedures in patients at least 18 years old.
In addition to standard saline implants, IDEAL IMPLANT Structured Saline Breast Implants are also available from The Waldorf Center. These advanced breast implant devices bridge the gap between saline and silicone implants, offering the best of both worlds and natural-looking results.
Special Financing Available
The Waldorf Center in Portland, OR, offers special financing for the recommended surgical or non-surgical procedure you want. Convenient monthly payments can be structured to fit your financial situation. Call the practice for more information at 503-646-0101 or contact us online.
THREE TALENTED DOCTORS
ONE FOCUSED MISSION
The experienced surgeons at The Waldorf Center for Plastic Surgery—Dr. Kathleen Waldorf, Dr. Rachel Streu and Dr. Heidi Johng—built the practice on a foundation of providing patients with high quality, ethical care. One of their guiding principles is a belief that cosmetic surgery is as much an art as it is a science.
The experienced surgeons at The Waldorf Center for Plastic Surgery—Dr. Kathleen Waldorf, Dr. Rachel Streu and Dr. Heidi Johng—built the practice on a foundation of providing patients with high quality, ethical care. One of their guiding principles is a belief that cosmetic surgery is as much an art as it is a science.
BREAST IMPLANT EXCHANGE
It’s not uncommon for women to want to upgrade their implants at some point. Breast implant exchange procedures can do just that. While advances in breast implants have made it possible for long-lasting and natural-looking results, the fact is that implants do not last forever, and it is necessary to have them changed out from time to time. Plus, there are other reasons women decide to exchange their implants, including:
To upgrade from saline to silicone or vice-versa
To increase or decrease breast size
To further enhance shape and projection of the breasts
To correct any complications or address dissatisfaction from a previous breast augmentation
Breast implant exchange procedures are often less complex and time-consuming than the original breast augmentation procedure. The same incisions can typically be utilized for implantation, leading to no additional scarring in other areas. Dr. Waldorf or Dr. Streu will talk with you about your goals and go over all of your options with you at the initial consultation.
Would you like more information about a Mommy Makeover for Portland, Vancouver, and all of Oregon at The Waldorf Center for Plastic Surgery? Call 503-646-0101 or 1-800-310-7901 to learn more, or schedule a visit online.
*Patient results may vary*